
Development of Cellular Medicines
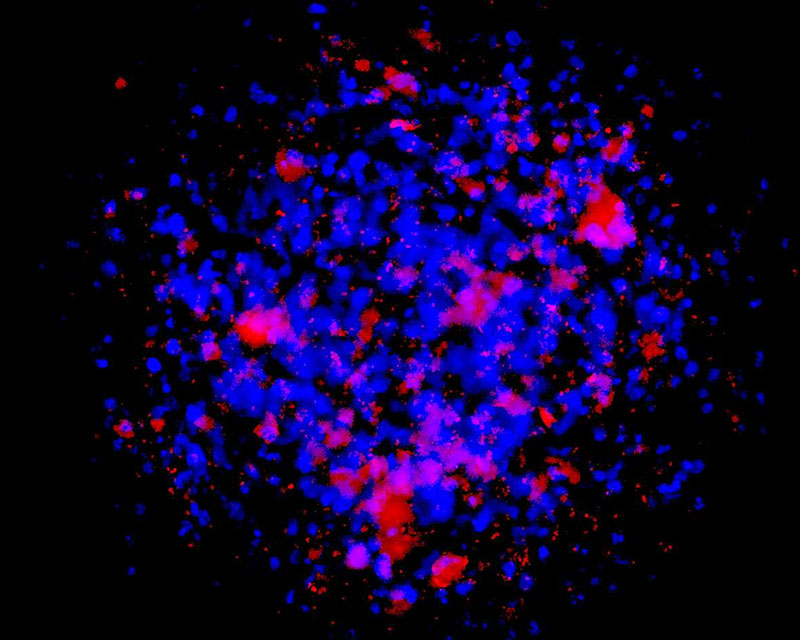
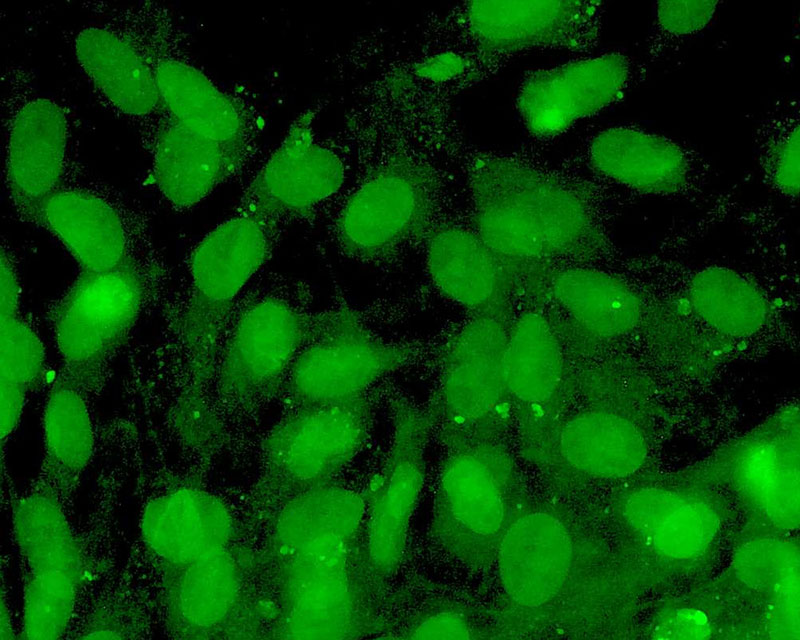
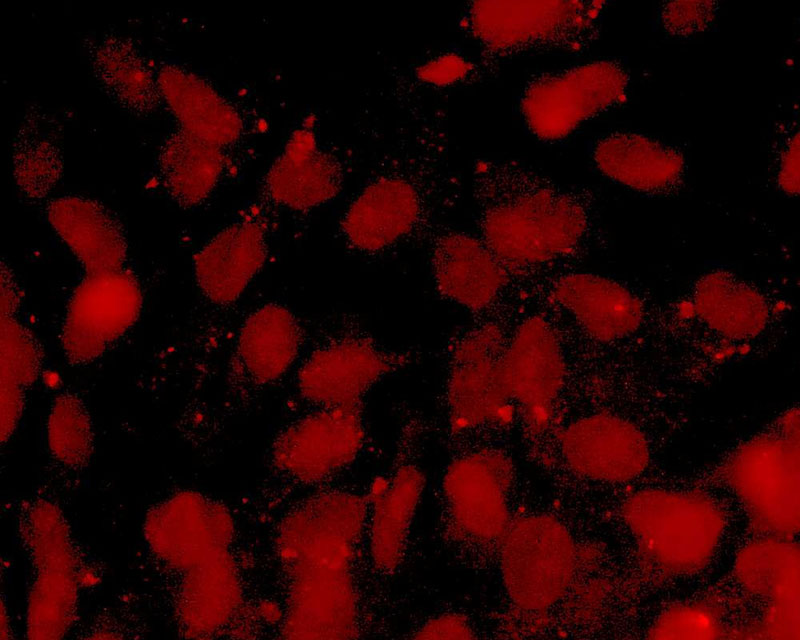
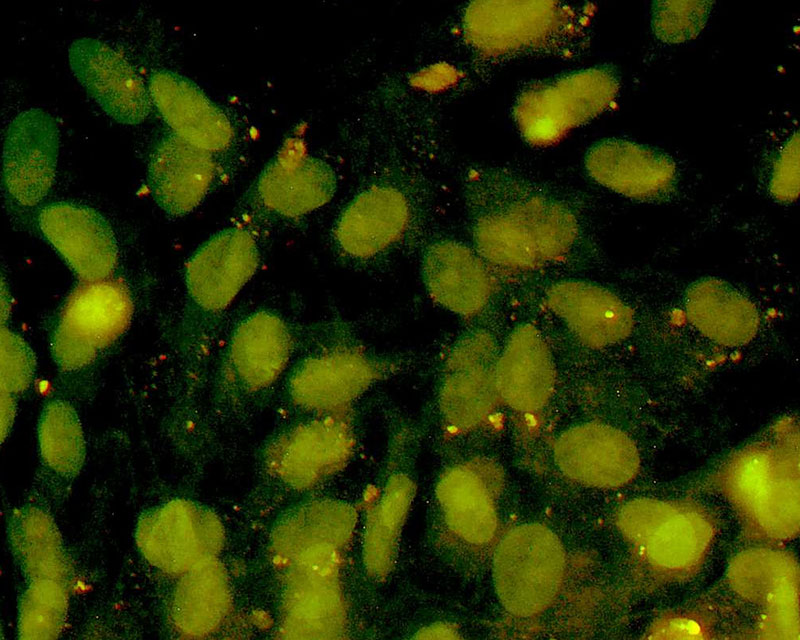
The development of a new method (patent pending) for the direct differentiation of somatic cells, such as fibroblasts, into other organ or tissue cells, has been achieved by the application of a newly developed CE-peptide.
This technology is called Direct Cell Reprogramming or Transdifferentiation because it does not use gene transfer methods such as those involved in iPS cell production, nor does it involve producing a pluripotent stem cell.
We hope to use this new technology to generate progenitor cells for organs from a patient's own cells and administer them as a medicine to repair damaged organs. For future commercialization, we intend to make progress in establishing preclinical proof of concept (POC) through collaborative research with universities and medical institutions, and to promote clinical implementation through collaboration with companies.
Cancer Immuno-Cell Therapy
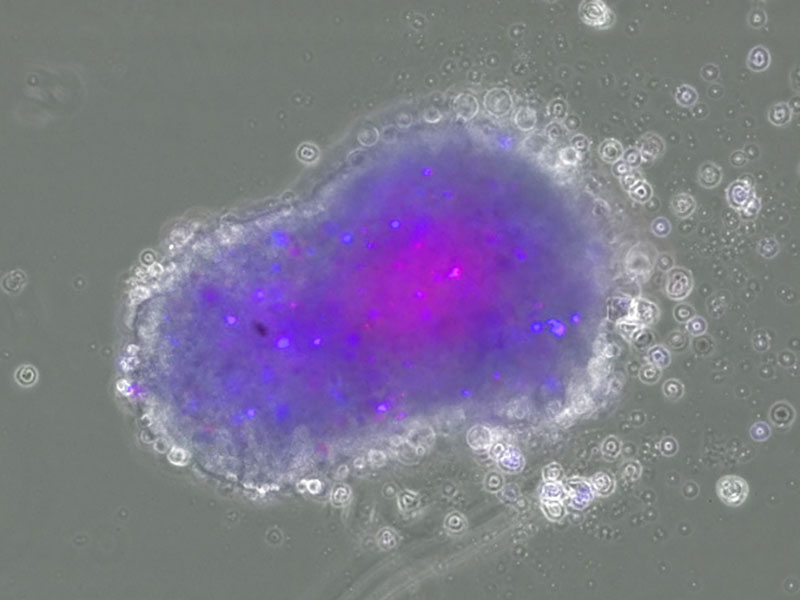

We are developing an exploratory model of neoantigens by integrating our CE-peptide and cancer stem cell organoid production technologies. Our objective is to develop applications for T-cell infusion therapy (TIL therapy, TCR-T therapy) in cancer immunotherapy, or cancer vaccine therapy using Neoantigen.
For future medical implementation, we intend to establish medical POC through joint research with medical institutions, research institutes, and universities working on tumor immunotherapy, as well as through collaboration with business companies.
